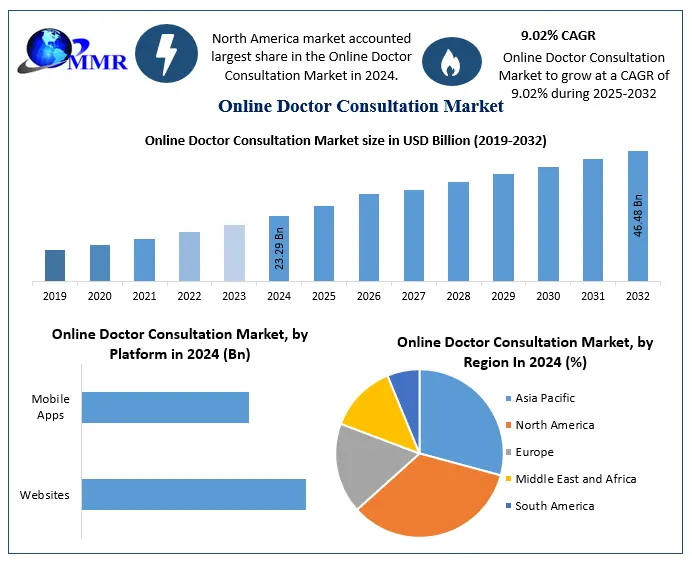
Clinical Trials Market Overview
The Clinical Trials Market is experiencing significant growth, driven by the increasing demand for innovative therapies and treatments. Valued at approximately USD 54.83 billion in 2024, the market is projected to reach nearly USD 88.26 billion by 2032, growing at a CAGR of 6.13%. This expansion is fueled by advancements in personalized medicine, the adoption of decentralized and virtual trial methodologies, and the integration of digital tools and telemedicine to enhance patient recruitment and data collection. The COVID-19 pandemic accelerated the shift towards remote trial methodologies, fostering greater acceptance of decentralized approaches and highlighting the need for more efficient and patient-centric trial processes.
Key players such as IQVIA, PPD, Parexel International, and Charles River Laboratories have been instrumental in shaping the clinical trials landscape through strategic initiatives, collaborations, and technological integrations. Regulatory advancements, including the FDA’s acceptance of real-world evidence, have also played a crucial role in transforming market dynamics. The emphasis on patient-centric approaches, coupled with investments in AI and data analytics, is poised to further revolutionize the clinical trial landscape, making trials more accessible, streamlined, and effective in bringing novel therapies to market.
Clinical Trials Market Research Methodology
The research methodology employed in analyzing the clinical trials market involves a comprehensive approach, incorporating both qualitative and quantitative analyses. This includes the examination of market dynamics, such as drivers, restraints, opportunities, and challenges, to provide a holistic understanding of the market landscape. Data collection is conducted through primary and secondary research methods, including interviews with industry experts, surveys, and the analysis of company reports, press releases, and regulatory filings.
Market segmentation is performed based on various parameters, including phase, study design, indication, service type, sponsor, application, and end-user. This segmentation allows for a detailed analysis of specific market segments and their respective growth trajectories. Furthermore, the research incorporates regional analyses to identify market trends and opportunities across different geographies. The combination of these methodologies ensures a robust and accurate representation of the clinical trials market, facilitating strategic decision-making for stakeholders.
Request a Sample of the US Tariff Impact Analysis Report:https://tinyurl.com/ncnj8kny
Demand & Supply Dynamics In Clinical Trials Market
The demand for clinical trials is escalating, driven by the increasing prevalence of chronic diseases, the need for personalized medicine, and the continuous development of novel therapies. This surge in demand necessitates a corresponding supply of clinical trial services, including patient recruitment, data management, and regulatory compliance. The adoption of decentralized and virtual trials has enhanced the supply capabilities by enabling remote monitoring and data collection, thereby improving efficiency and reducing costs.
However, the supply side faces challenges such as stringent regulatory requirements, the need for technological infrastructure, and the recruitment of diverse patient populations. To address these challenges, stakeholders are investing in advanced technologies, expanding their global research networks, and forming strategic partnerships. These efforts aim to streamline clinical trial processes, enhance patient engagement, and ensure compliance with regulatory standards, thereby balancing the demand and supply dynamics in the clinical trials market.
Clinical Trials Market Mergers & Acquisitions and Recent Developments
The clinical trials market has witnessed significant mergers and acquisitions, reflecting the industry’s drive towards consolidation and expansion. For instance, Thermo Fisher Scientific’s acquisition of CorEvitas, LLC for USD 912.5 million in August 2023 exemplifies strategic efforts to enhance service portfolios and cater to a larger patient pool. Such acquisitions enable companies to integrate advanced facilities, achieve synergies in capabilities and resources, and enhance their competitiveness in the market.
Recent developments also include the launch of innovative programs and collaborations aimed at improving clinical trial methodologies and patient engagement. For example, Parexel’s Community Alliance Network, launched in June 2022, integrates clinical research into community healthcare settings to serve patients better and increase diversity in clinical trials. Additionally, the adoption of self-collection safety lab panels and partnerships with technology companies are revolutionizing data collection and patient monitoring processes. These developments signify a dynamic and evolving clinical trials market, characterized by strategic collaborations and technological advancements.
For Customization of the report, please refer to this link:
Competitive Landscape Of Clinical Trials Market
The competitive landscape of the clinical trials market is characterized by the presence of prominent players such as IQVIA, Parexel International Corporation, Charles River Laboratories, ICON plc, and Syneos Health. These companies leverage significant experience and advanced technology to maintain their market positions. Phase III trials hold the largest market share due to their vital role in drug approval processes. North America leads the market, followed by Europe and the Asia-Pacific region.
New entrants in the market focus on micro-segments to bypass direct competition and often pursue partnerships, mergers, and acquisitions to establish their presence. The market is also witnessing increased investment in advanced technologies, such as AI, big data analytics, and remote monitoring, which provide companies with a competitive edge. Furthermore, the emphasis on patient-centric approaches and the integration of digital tools are reshaping the competitive dynamics, fostering innovation, and enhancing the efficiency of clinical trials.